Nanobody targeting intimin receptor inhibits the attachment of EHEC to human colonic mucosa.
- lagacetainfecciosa
- 2 dic 2019
- 2 Min. de lectura
Some strains of Enterohemorrhagic E.coli (EHEC) such as the serotype EHEC O157:H7, are a major public health concern in many countries causing diarrhoea, haemorrhagic colitis and haemolytic uremic syndrome (HUS) damaging kidneys and the central nervous system. However, nowadays we do not have specific vaccines or therapies available to prevent or treat immediately the pathology associated to these infections.
The article by the group of Dr. Fernández at the CNB is focusing on how to inhibit the attachment of EHEC to enterocytes, and in general to the mucosa of the colon. For this, the bacteria have a system based on two main proteins called intimin (Int) and the translocated intimin receptor (Tir). The mechanism of bacteria attachment is based on the secretion of effectors proteins that contribute to pathogenicity and cell damage through type III secretion system (T3SS). At the same time, it is also introduced the Tir (receptor), which will help to the adherence of intimin and it is essential for the intimate bacterial attachment, Tir clustering, and the polymerization of actin pedestals and therefore attaching/effacing lesions (A/E lesions).
In order to inhibit bacteria attachment, the authors propose a nanobodies-base system. The nanobodies (produced by camelids) are heavy-chain-only Abs where the antigen-binding site is formed by a single variable domain named VHH. The VHH have complementary determining regions (CDRs) capable of adopting specific conformations and recognise epitopes located in non-accessible protein cavities such as the active site of enzymes or inner regions of surface proteins from pathogens. They also show reversible folding properties and high resistance to proteolysis and thermal degradation.
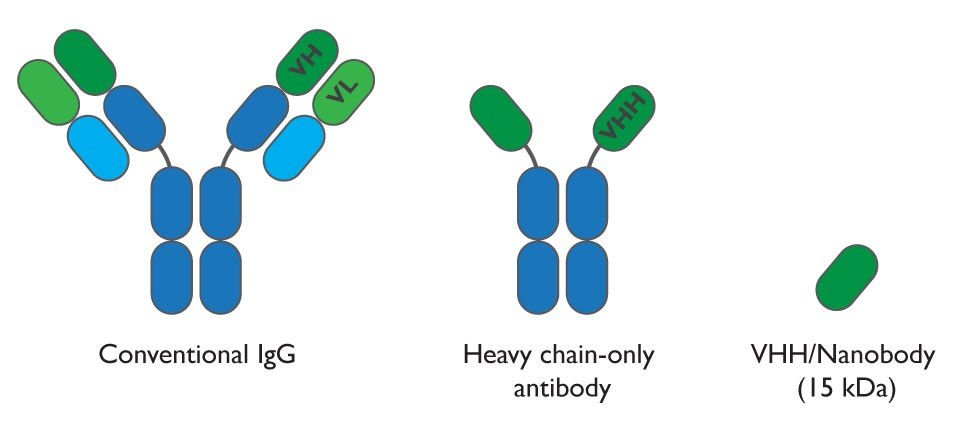
Another key virulence factor of EHEC are phage-encoded Shiga toxins (Stx) which are released into the bloodstream causing hemolytic uremic syndrome (HUS). However, application of antibiotics is discouraged because it induces Stx expression and therefore an increase of risk of developing HUS.
In a recent work published by Ruano-Gallego et al. in the magazine Plos Pathoges, the authors isolate Nbs capable to bind into specific regions of the receptor Tir. The results show that the only Nb with a high impact is the one which binds to TirM domain (a specific region of Tir) and is called TD4.
It has been proofed that TD4 reduces interaction of TirM with intimin (which is its natural ligand), by having higher affinity for it; then it also interferes with actin pedestal formation necessary for attachment of EHEC to human cells. They also demonstrate that TD4 treatment in a system of in vitro organ cultures (IVOC) can also inhibit attachment of EHEC to human colonic tissues. Finally, the authors proofed that if TD4 is introduced, after initial infection, the treatment with TD4 has inhibitory activity even once infection has begun.
Finally, in my opinion this method to prevent infections by Enterohemorrhagic E.coli has a lot of potential because it can be administered orally due to Nbs present resistance to gastric environment. However, the use of purified antibodies is a costly strategy for therapy development. To overcome this problem, some studies describe the production of Nbs in edible plants and seeds allowing oral passive immunization at the gastric mucosa surface; or the use of probiotics could be a way of delivering TD4 to the gastrointestinal tract.

Bibliography: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6738647
By Vicent Tur Planells. Microbiology course, 2º year of Biotechnology.


Comentarios